BY Malik Fahad Jjingo
HEJNU member in MASAKA
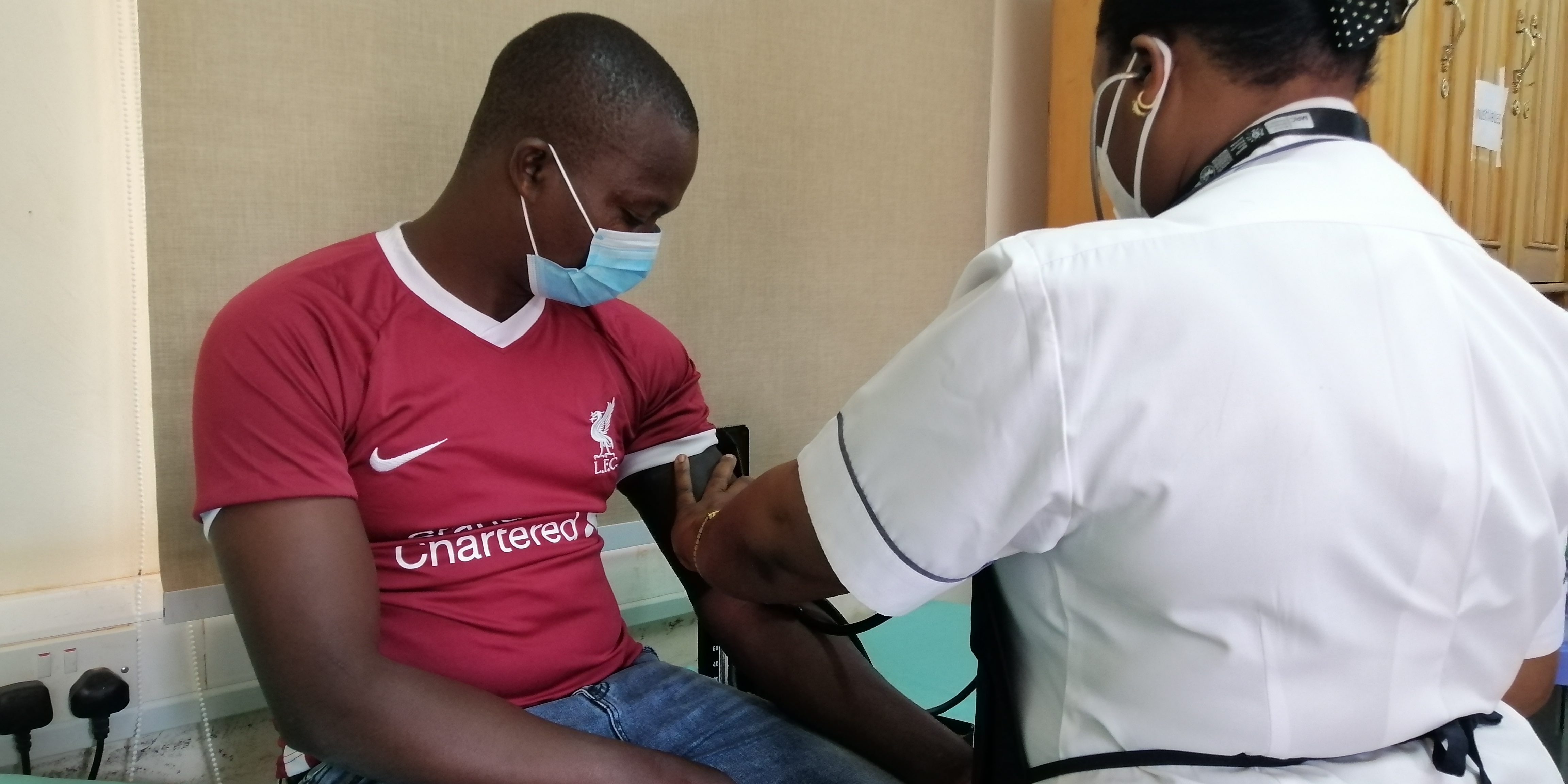
On Tuesday at 13;56 a volunteer in Masaka City received a shot in the arm and became one of the first of the 1,600 volunteers to participate in a massive African-led HIV vaccine trial.
The trial which will take place in four African countries of Uganda, Tanzania, South Africa, and Mozambique began at the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine (MRC/UVRI and LSHTM) Uganda Research Unit in Masaka.
The PrEPVacc study will test two HIV vaccine regimes and at the same time offer participants a new form of daily oral pre-exposure prophylaxis(PrEP) – descovy – which will be tested against the existing standard for PrEP – Truvada.
One vaccine regime combines DNA with the protein-based vaccine, and the other combines DNA, MVA, and protein-based vaccine. A section of participants will receive a placebo and others will receive a vaccine.
Prof. Pontiano Kaleebu, the director of MRC/UVRI and LSHTM Uganda Research Unit and the PrEPVacc Principal Investigator told reporters at MRC Masaka center that this vaccine trial provides two great opportunities to Africa; it is a milestone that Africans are taking part in the first HIV Prevention trial to test two ways to prevent HIV, a scourge that has ravaged the continent and the entire globe.
“The trial will give Africa an opportunity to grow the capacity of African sites to do future trials themselves and to foster their own future leaders,” he said.
The findings from PrEPVacc will inform scientists as to whether developing either of the two different combination vaccine regimens for preventing HIV is worthwhile or not. It will also inform them if descovy is as acceptable, safe, and effective as Truvada in women as well as men.
The vaccine trial will be conducted among healthy male and female volunteers aged 18-40 years and those likely to be at risk of HIV. As part of the trial, the participants will have scheduled clinic visits to the research center, undergoing HIV testing, and provide human samples – blood, urine etc
Prof. Jonathan Weber, Dean of the faculty of Medicine at Imperial College London, UK, which is supporting the PrEPVacc trial said that the trial participants in Masaka are helping communities and the world by answering important questions about how the researchers can best prevent HIV in future.
“These volunteers are critical to the success of PrEPVacc. We would not be in a position to ask these questions without the efforts of many other participants and researchers in the past,” said Weber. “I am immensely proud that we now have this African-Led European supported trial beginning in Uganda” he added.
PrEPVacc builds on a long history of partnerships between African countries and European institutions, including EuroVacc, AfrEVacc, TaMoVacc, and MDP.
Prof Sheena McCormack, PrEPVacc Project lead, based at the MRC Clinical Trials Unit at University College London, said PrEPVacc is a highly efficient and innovative study. “While we are testing two different ways to prevent HIV at the same time, we are also using a novel trial design that means that as the trial progresses, we can potentially spot where to save time and resources and focus on testing the vaccine regimens with the highest chance of success.”
“PrEPVacc is also addressing an important question about PrEP and its results will be valuable for informing future implementation and uptake strategies by local stakeholders and champions across East and Southern Africa where PrEp uptake is currently low,” he added.
Dr. Eugene Ruzagira a senior scientist and chief advisor of the project said that though this long journey started way back when they identified volunteers they are happy that it has started.
“The vaccine is safe and we are happy that we finally are able to start the trial that we have prepared for so long and we are hoping that the vaccination will produce efficacy as we hope” he added. He however said that the trial is going to be conducted amidst Covid19 which is a challenge.
The Masaka research center like all other facilities around the world has been affected by the pandemic which affected the commencement of the trial pushing its end date past the anticipated March 2023.
PrEPVacc is funded by the European and Developing Countries Clinical Trials Partnership (EDCTP), as part of the EDCTP2 program supported by the European Union. The Vaccine trial is led by African researchers from MRC/UVRI AND LSHTM Uganda research unit.
Other Research Units coordinating the study are at Mbeya, Tanzania, Dar –es –Salaam, Tanzania, Maputo, Mozambique, and Durban, South Africa. This will be the first HIV efficacy trial ever to be conducted in East African Countries. The total number of participants across all sites are a minimum of 1,668 participants.
ends