Recently approved malaria vaccines have reinvigorated hopes of eliminating the disease, but other innovations are in development that could further bolster these efforts.
- 12 March 2024
by Linda Geddes
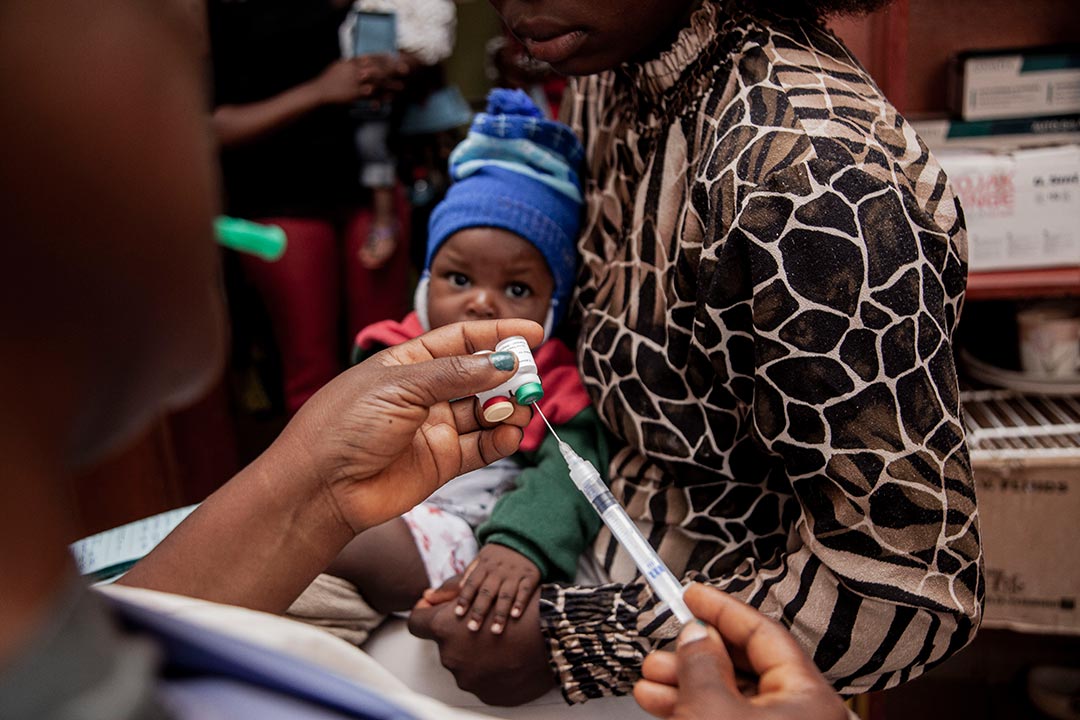
Malaria is one of the greatest infectious killers in human history and, despite recent control efforts, an estimated 600,000 people still die from it each year. The recent approval of the RTS,S and R21 vaccines have reinvigorated hopes of eliminating malaria, but these vaccines are not perfect, and we will need more than one tool to defeat the disease.
“Some studies are planning to look at a way of using RTS,S with a reduced dose, which could make it even more cost-effective. There are also questions about how best to implement the schedules of immunisation, particularly in settings where malaria is highly seasonal, of which there are many in sub-Saharan Africa.”
– Dr Larry Slutsker, former director of PATH’s Malaria and Neglected Tropical Diseases Program
Fortunately, other innovations are in the pipeline that could further bolster efforts to create a malaria-free world.
1. Optimisation of existing malaria vaccines
The scale-up and roll-out of vaccination campaigns involving the RTS,S and R21 vaccines will gain momentum in the coming months, but ongoing research is exploring the best dose and delivery strategy, particularly for the RTS,S vaccine.
“Some studies are planning to look at a way of using RTS,S with a reduced dose, which could make it even more cost-effective. There are also questions about how best to implement the schedules of immunisation, particularly in settings where malaria is highly seasonal, of which there are many in sub-Saharan Africa,” said Dr Larry Slutsker, a consultant to PATH and former director of its Malaria and Neglected Tropical Diseases Program.
For instance, is it better in areas where malaria is highly seasonal to vaccinate children when they reach a certain age, or should we deliver the initial series in the months just before the high transmission season, or indeed use a hybrid approach that combines both approaches?
“There’s also the question of whether you could mix doses of the two vaccines, so begin implementation with one vaccine and then switch to the other as you have access to it in real time,” Slutsker said.
2. Next-generation vaccines
The RTS,S and R21 vaccines target the malaria parasite as it enters the human body and travels to the liver, before it infects liver cells and replicates inside them. But this strategy relies on clearing every parasite: “If one gets into the liver and you have no blood-stage immunity, you get malaria,” said Prof Adrian Hill, chief investigator of the R21/Matrix-M programme and director of the Jenner Institute at the University of Oxford.
However, vaccines targeting other stages of the parasite’s lifecycle are being developed. These include blood-stage vaccines that target the parasite during its most destructive stage, when it undergoes further cycles of replication inside red blood cells, which is also when malaria symptoms occur.
Another approach is inducing antibodies to prevent parasites from maturing inside a mosquito after it has fed on an infected person. While such vaccines wouldn’t necessarily prevent the vaccinated person from being infected, they could reduce the number of infections in a community.
Assuming they can be made to work, combining different types of malaria vaccines could further enhance their efficacy. For instance, if a child received the R21 or RTS,S vaccine plus a blood-stage vaccine, then any parasites that did manage to infect the liver could be mopped up as they tried to infect blood cells.
Currently the two approved vaccines only target the Plasmodium falciparum parasite, the deadliest cause of malaria that is mainly limited to sub-Saharan Africa. Vaccines are also being developed against Plasmodium vivax, the dominant malaria parasite in the rest of the world.
3. Monoclonal antibodies
Monoclonal antibodies have transformed the way we prevent and treat various diseases, from cancer to childhood viral infections. They are lab-produced versions of the proteins that the body naturally uses to defend itself against disease, but these ones have been designed to attach to specific molecules, such as proteins found on the surface of the malaria parasites.
Injected into the body, such antibodies could provide potent protection against infection or transmission of malaria, but because they wouldn’t train the immune system to remember what the parasite looks like, as conventional vaccines do, such protection would not be long-lasting.
“All first-generation bed nets are based on pyrethroid insecticides, and there’s now a substantial amount of pyrethroid resistance out there in some countries. So, now there are dual ingredient nets being deployed, which contain other insecticides, and look like they are achieving good impact in areas where there’s a lot of pyrethroid resistance.”
– Dr Larry Slutsker, consultant to PATH
Even so, they could be useful in situations where someone requires robust protection for a relatively short period, such as during pregnancy. Other examples could include if someone is recovering from another illness that puts them at high risk of death if they catch malaria, or for travellers to malaria-endemic countries.
No monoclonal antibodies have yet been approved for malaria prevention, but a recent clinical trial suggested that a single dose of an antibody that neutralises malaria parasites before they infect liver cells protected healthy, non-pregnant adults from infection during an intense six-month malaria season in Mali, Africa.
4. Mosquito control
Malaria is transmitted by mosquitoes and most of the reduction in disease burden over the past two decades has been through the control of these vectors. Even with the new vaccines, bed nets will continue to provide the mainstay of protection over the near- to mid-, or even long-term.
These nets are now being tweaked and improved, Slutsker said. “All first-generation bed nets are based on pyrethroid insecticides, and there’s now a substantial amount of pyrethroid resistance out there in some countries. So, now there are dual ingredient nets being deployed, which contain other insecticides, and look like they are achieving good impact in areas where there’s a lot of pyrethroid resistance.”
Insecticides are also being developed that could be sprayed on the walls of people’s houses. In the research stage are new tools such as spatial repellents – devices that emanate a compound that repels mosquitoes from the surrounding area – and attractive targeted sugar bait (ATSB) devices that lure mosquitoes to feed on poison-laced sugar. “Mosquitoes need sugar meals as well as blood meals, and if they feed on the sugar and die, then the malaria parasite doesn’t have enough time to mature in the mosquito and be passed on to people,” said Slutsker.
Further ahead, so-called gene-drive mosquitoes could be a powerful tool in malaria elimination, if the public is willing to accept them. Various researchers are using the gene-editing technology CRISPR to introduce a modification that spreads through mosquito populations and makes female mosquitoes sterile. Also being investigated is whether infecting mosquitoes with a bacterium called Wolbachia could limit their ability to transmit malaria, by inhibiting the development of the malaria parasite within their bodies. Trials of both technologies are ongoing.
5. Next-generation antimalarial drugs
Artemisinin-based combination therapies (ACTs) have been a mainstay of malaria treatment over the past 20 years. They combine a derivative of artemisinin – a drug isolated from the sweet wormwood plant, which swiftly reduces the number of Plasmodium parasites in people’s blood – with a partner drug that eliminates the remaining parasites.
The problem is that the parasites are increasingly developing resistance to these drugs. While alternatives are in development, researchers are also deploying molecular methods to better understand what types of resistance are circulating in populations and tailoring drug combinations to specific groups or individuals within that population.
“The idea is to overwhelm the parasite by having multiple first-line therapies that you rotate or switch between,” said Slutsker. The theory is that when more than one drug is employed in a population at any given time, the emergence and spread of resistance may be delayed.
This article was originally published on
VaccinesWork
